Abstract
Juvenile myelomonocytic leukemia (JMML) is a rare myeloproliferative/myelodysplastic disorder with a poor prognosis. Unlike many other hematologic malignancies, it remains challenging to rapidly diagnose, and levels of residual disease are challenging to measure. Ongoing efforts to characterize the pathophysiology of JMML have yielded significant insight, but a complete picture of the cellular hierarchy of the disease has been elusive.
To obtain an unsupervised view of the heterogeneity of hematopoietic cell types in JMML, we performed single-cell RNA multiomics on serial samples from a 4-year-old patient who presented with fevers, hepatosplenomegaly, leukocytosis, monocytosis, elevated Hemoglobin F, and bone marrow blast count of 7%. Clinical sequencing revealed mutations in PTPN11 (VAF 66%), NF1 (VAF 17%), MYB (VAF 12%), and SH2B3. We obtained a sample for multiomic profiling following his initial cycles of chemotherapy when PTPN11 variant frequency was 32%, and he remained critically ill. The subsequent sample (6 weeks later) was collected when no mutated PTPN11 was detectible, and his clinical picture had significantly improved. Both samples were processed using gradient centrifugation, and a subset of the cells captured was immunomagnetically enriched CD34+ cells. A panel of 141 oligonucleotide-tagged antibodies was used to label the cells prior to single-cell capture. The resulting libraries were sequenced using standard short-read sequencing, and reads were demultiplexed and counted using common workflows. Single-cell profiles were visualized using UMAP. Cells were annotated using a highly performant machine-learning algorithm that classifies cells using a healthy reference of human BM, which we have previously validated using publicly available data.
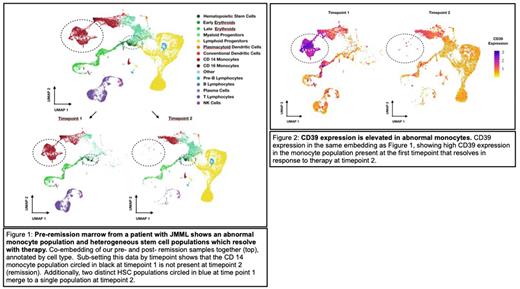
Examining all cells together, we observed changes in two key cell types over time (Figure 1). First, we noted a large population of monocyte-like cells with increased expression of genes associated with complement, inflammation (including IL1beta), and KRAS signaling in the pre-remission sample, which nearly completely disappeared in the second sample. Moreover, surface expression of CD39 was significantly higher in these cells than in all other cells measured (Figure 2). These data suggest CD39 may represent a novel biomarker to monitor treatment response in JMML or as a potential target for therapy. Second, we observed two distinct hematopoietic stem cell (HSC) populations in the pre-remission sample and only one in the remission sample. Differential gene expression analysis between the two pre-remission HSC clusters revealed enrichment of specific genes previously associated with JMML progenitors in one, including HOPX. This population of HSCs also exhibited higher expression CD7, which correlated with the patient's flow cytometric data from the same time point. We hypothesize that CD7/HOPX expressing HSCs in the first sample were JMML progenitor cells eliminated by therapy.
Our work demonstrates the power of single-cell multiomics to dissect cellular heterogeneity in diseases with complex clonal hierarchies. Moreover, these data can also be a resource for identifying potential biomarkers and drug targets. Specifically, our data suggest that inflammatory monocytes may be a distinct target for therapy, which may benefit critically ill patients like ours. Future efforts will be focused on profiling additional patients and rigorously characterizing the mutational state of individual cells.
Disclosures
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal